Synaptic adhesion molecule IgSF11
Synaptic adhesion molecules regulate synapse development and plasticity through mechanisms that include trans-synaptic adhesion and recruitment of various synaptic proteins. We discovered that immunoglobulin superfamily member 11 (IgSF11), a homophilic adhesion molecule that is preferentially expressed in the brain, is a double-bond partner of postsynaptic scaffold protein PSD-95 and receptors AMPA from glutamate (AMPAR).
IgSF11 required PSD-95 binding for its excitatory synaptic localization. In addition, IgSF11 stabilized synaptic AMPARs, as determined by IgSF11-knockdown-induced suppression of AMPAR-mediated synaptic transmission and increased surface mobility of AMPARs, as measured by high-throughput tracking d a single molecule.
IgSF11 deletion in mice resulted in suppression of AMPAR-mediated synaptic transmission in the dentate gyrus and long-term potentiation in the CA1 region of the hippocampus. IgSF11 did not regulate the functional characteristics of AMPARs, including desensitization, deactivation, or salvage. These results suggest that IgSF11 regulates excitatory synaptic transmission and plasticity through its tripartite interactions with PSD-95 and AMPARs.
The most important structural feature of mammalian neocortical circuitry is the layered organization of specific cell types and synaptic inputs. Accordingly, cortical inhibitory interneurons (INs), which shape local network activity, exhibit subtype-specific laminar specificity of synaptic outputs. However, the underlying molecular mechanisms remain unknown.
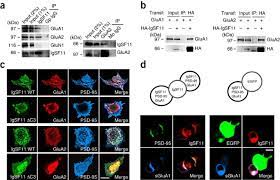
Here, we demonstrate that homophilic adhesion proteins from immunoglobulin superfamily member 11 (IgSF11) are preferentially expressed in one of the most distinctive IN subtypes, namely luster cells (ChCs) that innervate specifically the initial segments of the axons of pyramidal neurons (PN) and their synaptics.
laminar target. Loss-of-function experiments in ChCs or post-synaptic cells revealed that IgSF11 is required for synaptic development of ChCs in the target layer. Whereas overexpression of IgSF11 in ChCs enlarges the presynaptic knobs of ChC, expression of IgSF11 in non-target layers induces ectopic synapses of ChC. These results provide evidence that synapse-promoting adhesion proteins, highly localized to synaptic partners, determine layer-specific synaptic connectivity of the cortical IN subtype.
The mammalian neocortex is organized into six distinct cytoarchitectural layers. Each layer contains specific types of excitatory pyramidal (PN) neurons that are defined by gene expression, morphology, electrophysiological property, and distance projection targets (1, 2). PNs in distinct layers receive inputs from a different set of intracortical and subcortical neurons, generating specific output signals (3–8). Among these multiple sources of inputs to PRs, cortical inhibitory interneurons (INs) locally innervate PRs and play a key role in shaping their activity at the synaptic, cellular and network levels (9-12).
They exhibit a high degree of diversity in cellular properties such as morphology, physiology, gene expression, and connectivity (9-11). Recent studies have shown that distinct IN subtypes are functionally specialized (13–15). Thus, assembly of inhibitory inputs from a specific combination of IN subtypes is likely one of the crucial steps in constructing layer-specific computational modules.
Individual IN subtypes exhibit one or a few preferential synaptic target layers (6, 14, 16-19), suggesting that they are assigned to participate in layer-specific information processing. For example, somatostatin-positive (SOM+) Martinotti cells in deeper layers predominantly innervate the apical dendrites of PNs in layer 1 (L1), where information from intracortical and subcortical neurons is integrated (6, 14). One type of fast-spiking L4 parvalbumin-positive (PV+) IN forms reciprocal connections with excitatory spiny neurons in the same layer (19).
The assembly of layer-specific local networks comprising INs and PNs involves different developmental events, including cell migration, laminar positioning, axonal morphogenesis, and synapse formation (11, 20-22). Extensive evidence has indicated that cell type-specific interactions between INs and PNs contribute to at least some of these developmental processes.
Corticofuge PNs converting fate to callosal PN in L5 by manipulating a master transcriptional regulator reduce the number of L5 PV+ and SOM+ IN, which are normally enriched in deep cortical layers (21). Genetic reprogramming that changes the fate of callosal PNs to corticofuge PNs in L2/3 induces more perisomatic buttons of PV+IN on fate-converted PNs at the level equivalent to endogenous L5 corticofuge PNs (20).
These results suggest the cellular extrinsic mechanisms that mediate cell type-specific communications between INs and PNs during assembly of layer-specific circuit modules. However, little is known about the molecular mechanisms underlying synaptic pairing between INs and their target layer PNs.
