CaM Kinase II-enzyme
CaM kinase II or CaMKII, also called Ca2+/Calmodulin-dependent protein kinase II, is a serine/threonine-specific protein kinase that is regulated by the Ca2+/calmodulin complex.
CaMKII is a family of multifunctional protein kinases that activate signaling pathways. CaMKII is involved in numerous signaling cascades and is considered an important mediator of learning and memory. CaMKII downstream targets that may be involved in the regulation of neuronal viability. CaMKII signaling can promote excitotoxic cell death or neuronal survival. Activation or inhibition of downstream targets is indicated. Some, but not all, of these downstream effects are mediated by direct phosphorylation of the target.
CaMKII has emerged as a key nodal protein in the regulation of cardiac physiology and pathology. CaMKII misregulation is linked to Alzheimer's disease, Angelman syndrome, and cardiac arrhythmia. CaMKII is also required for Ca2+ homeostasis and reuptake in cardiomyocytes, chloride transport in epithelia, positive selection of T cells, and CD8 T cell activation.
Open AccessRevision
Physiological and pathological roles of CaMKII-PP1 signaling in the brain
by Norifumi Shioda 1,* and Kohji Fukunaga 2,*
Department of Biofunctional Analysis Molecular Biology Laboratory, Gifu Pharmaceutical University, 1-25-4 daigaku-nishi, Gifu 501-1196, Japan
Department of Pharmacology, Graduate School of Pharmaceutical Sciences, Tohoku University, 6-3 Aramaki-Aoba, Aoba-ku, Sendai, Miyagi 980-8578, Japan
Authors to whom correspondence should be addressed.
Int. J.Mol. Science. 2018, 19(1), 20; https://doi.org/10.3390/ijms19010020
Received: November 23, 2017 / Reviewed: December 19, 2017 / Accepted: December 20, 2017 / Posted: December 22, 2017
(This article belongs to the Kinase Signal Transduction 2017 special issue)
Download PDF Browse Figure Citation Export
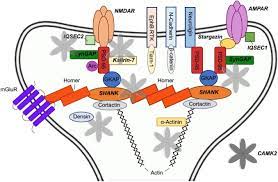
Ca2+/calmodulin (CaM)-dependent protein kinase II (CaMKII), a multifunctional serine (Ser)/threonine (Thr) protein kinase, regulates various activities related to Ca2+-mediated neuronal plasticity in the brain, including synaptic activity and gene expression. Among its regulators, protein phosphatase-1 (PP1), a Ser/Thr phosphatase, appears to be essential in the control of CaMKII-dependent neuronal signaling.
In postsynaptic densities (PSDs), CaMKII is required for hippocampal long-term potentiation (LTP), a cellular process correlated with learning and memory. In response to Ca2+ elevation upon induction of LTP in the hippocampus, CaMKIIα, an isoform that translocates from cytosol to PSDs, is activated by autophosphorylation at Thr286, generating autonomous kinase activity and a bound state to prolonged Ca2+/CaM. In addition, PP1 inhibition enhances Thr286 autophosphorylation of CaMKIIα upon LTP induction.
In contrast, the nuclear import of CaMKII is regulated by the phosphorylation state of Ser332. CaMKIIδ3, a nuclear isoform, is dephosphorylated at Ser332 by PP1, promoting its nuclear translocation, where it regulates transcription. In this review, we summarize the pathophysiological roles of CaMKII/PP1 signaling in neurons. CaMKII and PP1 crosstalk and regulation of gene expression are important for neuronal plasticity as well as survival and/or differentiation.
La CaM kinase II ou CaMKII, également appelée Ca2+/Calmodulin-dependent protein kinase II, est une protéine kinase spécifique à la sérine/thréonine qui est régulée par le complexe Ca2+/calmoduline.
CaMKII est une famille de protéines kinases multifonctionnelles qui activent les voies de signalisation. CaMKII est impliqué dans de nombreuses cascades de signalisation et est considéré comme un médiateur important de l'apprentissage et de la mémoire. Cibles en aval CaMKII qui peuvent être impliquées dans la régulation de la viabilité neuronale. La signalisation CaMKII peut favoriser la mort cellulaire excitotoxique ou la survie neuronale. L'activation ou l'inhibition des cibles en aval est indiquée. Certains de ces effets en aval, mais pas tous, sont médiés par la phosphorylation directe de la cible.
